How to buy bitcoin in Denmark



Denmark is included in the list of countries that have created a favorable environment for the development of bitcoin and other digital assets. Despite the fact that there are no official cryptocurrency regulators in the local legislative system, virtual currency trading is not prohibited in Denmark, and bitcoin is accepted as a payment instrument in many online and offline stores. This lack of government control gives to the users almost complete freedom of action.
To purchase bitcoin at the current market price for euros, dollars or other currencies, just go to the official exchange service Itez and go through all the steps step by step, following a simple instruction.
In order to buy bitcoin, it is not necessary to register on the Itez website. In a handy calculator, you can quickly calculate the cost of BTC based on the amount you plan to use for payment. The smallest purchase amount in dollar terms is 30 USD. By the way, the same applies to the euro - the starting purchase threshold starts at €30. Well, in our instructions, as promised, we will go through all the stages of purchase BTC for dollars step by step.
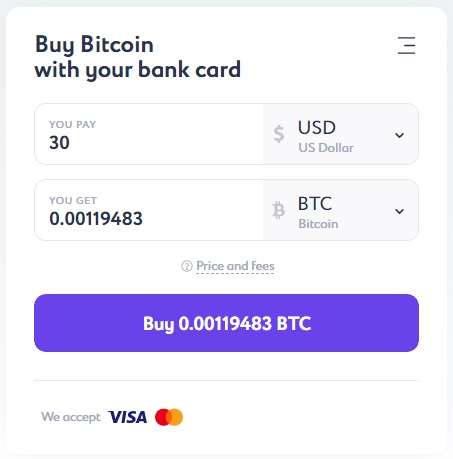
After converting the currency to BTC, you will see the final result in the "You Get" field and on the "Buy" button. Click the button and enter your email address in the following window.
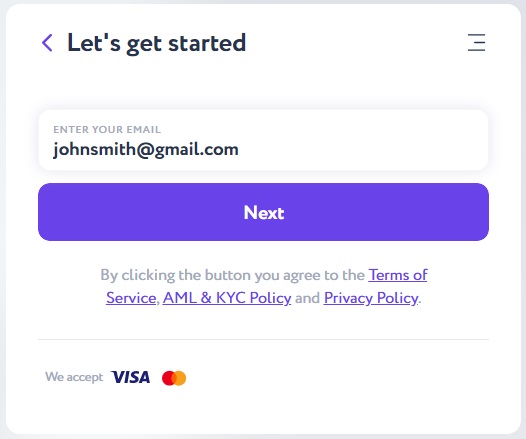
Verify your email with a 6-digit code sent to the previously indicated e-mail and click the "Verify" button.
Specify the purchase amount in the Itez dialog box. Copy the recipient address - your bitcoin wallet - from the “Accept” section.
Important: you should not manually copy the bitcoin wallet address. The blockchain system does not give any information about bitcoin addresses problems, and if an error occurs during manual entry, all the buyer's funds may be lost.
For example, this is how the copy address button looks like in the Exodus wallet:
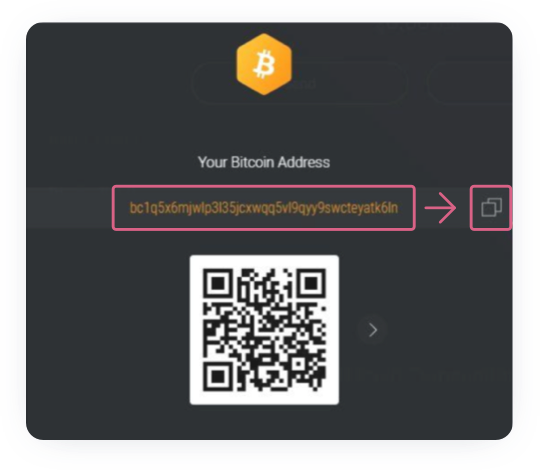
After copying the bitcoin wallet address, paste it into the dialog box.
Check your data — it is important to make sure that the address is entered correctly.
Don't have a crypto wallet?
Create your convenient and secure itez Wallet in just a few minutes!
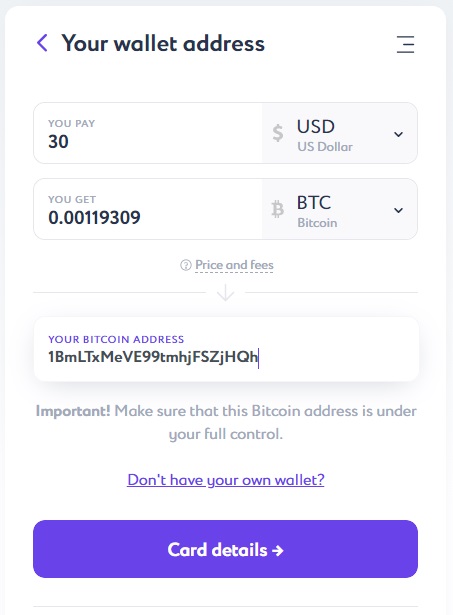
After pushing the “Card details” button, enter the required card information: card number and expiration date, the cardholder name and CVC-code — three numbers on the back of the card.
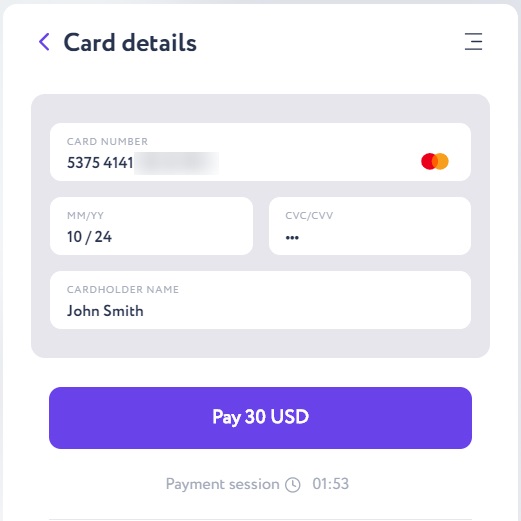
Push the “Pay” button and enter the one-time bank code in the following window. As soon as the transaction receives 6 confirmations in the blockchain network, your bitcoin wallet balance will be top-up.
Important: the transaction will wait for confirmations for time. After six network confirmations, bitcoin will be on your e-wallet; when buying with Itez it will take for about 15 minutes. This is a very good speed for the Bitcoin network since transactions in the most popular blockchain network are somewhat slower than in other networks due to its properties and high load.
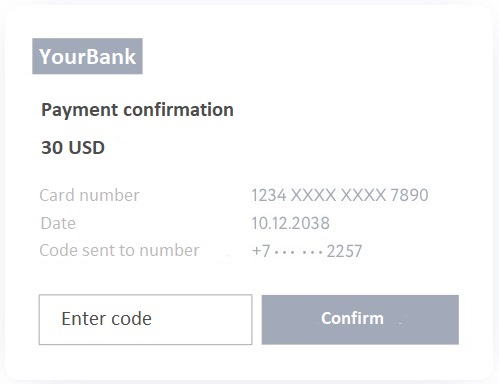
The transaction will first wait for confirmations from the blockchain network.
After six confirmations, the BTC networks will be yours for good; when buying from Itez, this will happen on average within 15 minutes.
This is a very good indicator for the Bitcoin network, since transactions are somewhat slower in the most popular blockchain network than in other networks, due to its design features and high workload.

The Danish banking system is focused on increasing the number of clients. This is facilitated by the creation of loyal conditions for the exchange and storage of cryptocurrencies and the absence of commissions when making transactions. The list of the most popular banks in Denmark includes Danske Bank A / S, Nykredit, Jyske Bank, Sydbank, Arbejdernes Landsbank and many others. A client of one of these and any other local bank can count on a high level of service when buying a cryptocurrency: not only the clients themselves are focused on this, but also the state, which seeks to promote the national currency and stabilize the financial systems.

Traditionally, to purchase cryptocurrency, almost all the users choose credit and debit cards of the international payment systems Visa and MasterCard. However the Dankort online payment service is also popular in Denmark. These financial institutions regularly check for compliance with the requirements for the implementation of the exchange process and allow transactions to be carried out around the world.

Statistics show that only 14% of Danish users are attentive to the protection of the purchased cryptocurrency. In order to ensure the safety of digital assets, you need to use a special electronic wallet. The advantages and disadvantages of popular electronic wallets for storing bitcoin are described in detail at bitcoin.org
Itez is an instant bitcoin purchase resource. Itez is a certified operator holding a license to sell and store cryptocurrencies of a financial regulator from Estonia. What does this give to customers?
First of all it is safety, since the operator is fully responsible for the risks. He conducts a compliance check, provides cryptocurrency on time and is always in touch with customers, if the need arises. Secondly, it`s efficient. The average time for receiving bitcoin to a client's account is a quarter of an hour. For comparison, let's take similar services Indacoin and MoonPay: when working with them, the time it takes for bitcoin to be credited to the client's electronic wallet will take for about an hour - it depends on how busy the network is. Thirdly, the service offers to customers a favorable exchange rate. Rate changes occur in accordance with the liquidity provider's information, therefore, the BTC value on the Itez website is as close as possible to the market value. The site has no hidden fees as the final BTC value is displayed instantly. If the purchase price does not exceed € 300, then the buyer does not need verification.